By : Dr Kirti Kajal, PhD Cancer Biology, Medical Writer
Cells function in homeostasis. To maintain the proper functioning of a cellular system there is always a balance between activators and inhibitors. Normally, in adults` angiogenesis occurs during wound healing and the reproductive cycle in a woman. Apart from these physiological conditions, if angiogenesis occurs then it is an indication of pathogenesis. Pathological angiogenesis occurs during cancer, and inflammatory diseases such as rheumatoid arthritis, diabetic retinopathy, macular degeneration, and endometriosis.
In solid tumors, such as breast cancer as cancer cells grow beyond 100mm-200mm in size, due to lack of blood supply, cells suffer from oxygen and nutrients deficiency. To fulfill their requirements cancer cells, secrete plethora of cytokines, chemokines, and other pro-angiogenic molecules. This disrupts the cellular ratio between angiogenic activators and inhibitors and initiates neo-angiogenesis.
The neovascularization vis-à-vis neo-angiogenesis facilitate cancer cell survival, sustenance, migration, and metastasis. It imparts aggressive and invasive phenotype to cancer cells.

The key players of neo-angiogenesis are
- Hypoxia inducing factor (HIF-1α)
- Vascular endothelial growth factor (VEGFA or VEGF, VEGFB, and VEGFC)
- Vascular endothelial growth factor receptors (VEGFR1, VEGFR2, and VEGFR3)
- Delta-like ligand (DLL)/Notch pathway
- Mitogen-activated protein kinase (MAPK) pathway
- PI3K/AKT/mTOR pathway
- matrix metalloproteases (MMPs)
- Angiopoietins.
To understand the neo-angiogenesis processes, we must know normal vascular biology, viz., vasculogenesis and angiogenesis.
VASCULOGENESIS
Vasculogenesis is a process of formation of the primary vascular plexus at the embryonic stage. Hemangioblasts (progenitor: embryonic stem cells) form hematopoietic and endothelial cells. The hematopoietic (inner cells) and endothelial cells (outer cells) form blood islands in the yolk sac.
The embryonic vessels such as the aorta and veins are formed by the fusion, differentiation, and proliferation of migratory angioblasts within an avascular tissue.
In adults, vasculogenesis initiates from bone marrow-derived circulating cells. The bone marrow derived circulating cells comprise of endothelial progenitor cells (EPCs), myeloid cells, mesenchymal, and lymphocytic cells.
ANGIOGENESIS
Angiogenesis is a process of formation of arteriolar network, venules, veins, and branched capillaries from embryonic vascular plexus. The arteries, veins, venules, and highly branched capillaries provide nutrients and oxygen to the tissues and organs. Angiogenesis comprises matrix degradation, sprouting, intussusceptive growth, splitting, lumen formation, endothelial cell survival, remodelling, and specialization & differentiation of endothelial cells into arterioles, venules, and capillaries.
Matrix degradation
Earlier, VEGF was known as vascular permeability factor (VPF). VEGF/VPF permeabilizes and disintegrates the matrix via redistribution of PECAM (platelet endothelial cell adhesion molecules)-1 and vascular endothelial cadherin.
Fibroblast growth factor (FGF)-2 and matrix metalloproteinases (MMPs, plasminogen activators) are intricately involved in sprout formation by facilitating matrix degradation.
Sprouting
Angiogenesis is a series of guided processes that involves the migration of proliferating tip cells and proliferation of stalk cells.
The VEGF and NOTCH gradient leads to the formation of tip cells and stalk cells. VEGF/VEGFR2, NOTCH/DLL4, and Jagged 1 are primarily associated with proliferating tip cell migration and stalk cell proliferation.
The leading tip cells migrate in response to VEGF stimulus while NOTCH/DLL4 and Jagged 1 regulate (initiate or inhibit) stalk cell proliferation. Besides, VEGF there are various other factors such as FGF, angiopoietin, etc., involved in the sprouting process.
Like VEGF, other forms of VEGF, FGF, and angiopoietin activate their downstream signaling pathways to sustain the sprouting process.
Lumen formation
The lumen formation involves two major processes- (a) Notch-mediated termination of tip cell migration and proliferation and (b) anastomoses of tip cells of two budding sprouts. This culminates the lumenization process between branches of the growing vascular plexus.
It has been reported that small GTPases (guanosine triphosphate) cell division control protein 42 (CDC42), integrin cell-matrix adhesion machinery, and Rac family small GTPase 1, Rac1(pinocytosis) are involved in lumen formation.
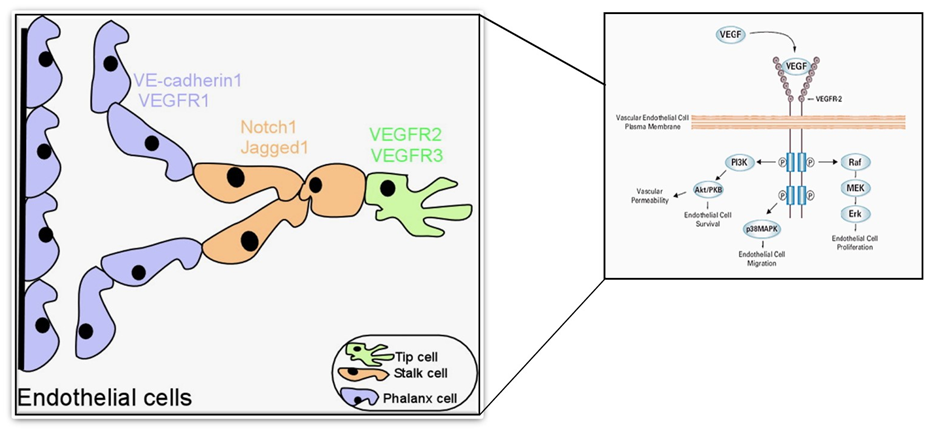
Endothelial cell survival
The activation of VEGF/VEGFR2 downstream signaling (shown in the figure above) is principally involved in endothelial cell survival during sprouting.
Maturation, remodeling & endothelial cell differentiation
The maturation and remodeling of endothelial cells require angiopoietins, Ang1, Ang2, Ang3, and Ang4. Ephrin-B2 (ephrin family proteins) are essential requirements for the differentiation of endothelial cells into evolving arteries and veins.
Ang-1, a ligand of Tie 2 (endothelial endothelium specific receptor tyrosine kinase) is associated with the tightening of blood vessels.
NEOVASCULARIZATION
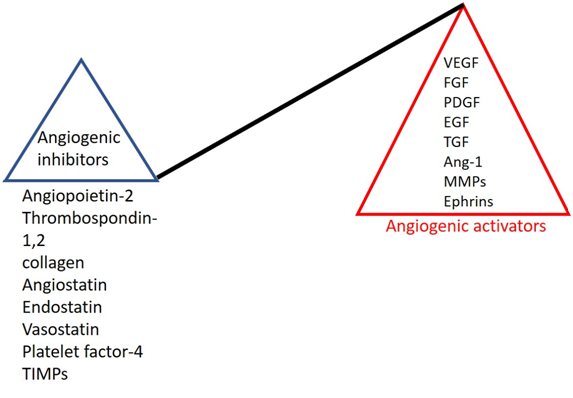
Hypoxia-inducible factor -1α induced transcription of VEGF, FGF, hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF) primarily leads to hypervascularization in the growing solid tumors. Normally, during wound healing, angiogenic activators are stimulated, and as the wound heals angiogenic activators are perturbed by angiogenic inhibitors.
In tumor microenvironment, the cellular ratio of angiogenic activators (VEGF family, FGF, PDGF, epidermal growth factor (EGF), transforming growth factor (TGF), angiopoietin-1, ephrin family, and MMPs) get increased by several folds as compared to angiogenic inhibitors (angiopoietin-2, thrombospondin-1,2, collagen, angiostatin, endostatin, vasostatin, platelet factor-4 and tissue inhibitor of metalloproteases (TIMPs)).
Cytokines-chemokines (induced by angiogenic activators) infiltrate different types of immune cells, such as T-regulatory cells, dendritic cells, mast cells, neutrophils, macrophages, natural killer (NK) cells, and B-cells also secrete VEGF—further increasing the level of VEGF in the tumor microenvironment. This uncontrolled hypervascularization causes increased vascular permeability, leaky blood vessels (facilitates tumor cell migration and metastasis to new sites), cell-tissue disruption, and organ failure.
The inability to detect these aggressive and invasive tumors at an early stage is the principal reason for the worldwide mortality caused by cancer.
Dual role of VEGF
VEGF, the principal angiogenic activator becomes the major wrongdoer in the tumor milieu. VEGF—apart from playing the key role in neovascularization, also act as an— immunosuppressor in the following ways:
- Inhibit immune cell recruitment on endothelial cells via suppressing the expression of adhesion molecules VCAM-1 and ICAM-1.
- Perturbs cytotoxic immune cells migration in the tumor region from lymph nodes (abnormal vasculature and leaky blood vessels).
- Depletion of cytotoxic CD8+T-cells: VEGF increases the expression of IL-2, PD-1, PDL-1 (dendritic cells) CTLA-4, LAG-3 protein, and T-cell immunoglobulin mucin receptor 3. Their engagement with effector T-cells causes T-cell anergy.
- Effector T-cell apoptosis: VEGF-led increased expression of FAS ligand on tumor endothelial cells causes apoptosis of effector T-cells.
- T-reg induction: VEGF-induced-FOXP3 and IL-2 (increased level) promote Treg induction in tumor microenvironment.
- Inhibition of antigen-presenting cells: VEGFR-1 and neuropilin-1 inhibit dendritic cell maturation. Immature dendritic cells lead to inactive cytotoxic T-cells.
- Induction of MDSCs (myeloid-derived suppressor cells): MDSCs promote tumor advancement\ by secreting VEGF and suppressing the activation of cytotoxic T-cells.
Conclusion
Cancer cells utilize the host machinery for its propagation and progression. Hence, curing cancer is the most challenging task for scientists, clinicians, and pharmacists. There are various drugs available in market that target VEGF/VEGFR (Avastin and SU5416), only VEGF (bevacizumab in combination with 5-fluorouracil, punarnavine, aflibercept, and acacetin), only VEGFR (celestrol), and combinatorial therapies targeting endothelial cells and FGF2 (fucoxanthin and siphonoxanthin, etc.). These drugs have side effects such as drug resistance, allergies, gastritis, etc, hence, they have not been translated into medicine.
Source
Gerhardt H. VEGF and Endothelial Guidance in Angiogenic Sprouting. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6141/
Stephen, N. M., Deepika, U. R., Maradagi, T., Sugawara, T., Hirata, T., & Ganesan, P. (2023). Insight on the cellular and molecular basis of blood vessel formation: A specific focus on tumor targets and therapy. MedComm – Oncology, 2(1), e22. https://doi.org/10.1002/mog2.22
Mühleder S, Fernández-Chacón M, Garcia-Gonzalez I, Benedito R. Endothelial sprouting, proliferation, or senescence: tipping the balance from physiology to pathology. Cell Mol Life Sci. 2021 Feb;78(4):1329-1354. doi: 10.1007/s00018-020-03664-y. Epub 2020 Oct 19. PMID: 33078209; PMCID: PMC7904752.
You WK, Schuetz TJ, Lee SH. Targeting the DLL/Notch Signaling Pathway in Cancer: Challenges and Advances in Clinical Development. Mol Cancer Ther. 2023 Jan 3;22(1):3-11. doi: 10.1158/1535-7163.MCT-22-0243. PMID: 36223541; PMCID: PMC9808372.
Vessel Sprouting – an overview | ScienceDirect Topics
Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J. 2010 Feb;6(1):85-94. doi: 10.1007/s11420-009-9129-4. Epub 2009 Sep 9. PMID: 19763695; PMCID: PMC2821499.
Kajal K, Bose S, Panda AK, Chakraborty D, Chakraborty S, Pati S, Sarkar T, Dhar S, Roy D, Saha S, Sa G. Transcriptional regulation of VEGFA expression in T-regulatory cells from breast cancer patients. Cancer Immunol Immunother. 2021 Jul;70(7):1877-1891. doi: 10.1007/s00262-020-02808-0. Epub 2021 Jan 4. PMID: 33394094.
Ribatti D. Immunosuppressive effects of vascular endothelial growth factor. Oncol Lett. 2022 Sep 1;24(4):369. doi: 10.3892/ol.2022.13489. PMID: 36238855; PMCID: PMC9494354.




